Chemical Reactions
A chemical reaction occurs when one or more reactants are changed into one or more products. The constituent atoms of the reactants are rearranged in a chemical reaction, resulting in the formation of various substances as products.
Physical and Chemical Changes
Chemical change – one or more new substances with new physical and chemical properties are formed.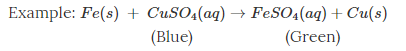
Here, when copper sulphate reacts with iron, two new substances, i.e., ferrous sulphate and copper, are formed.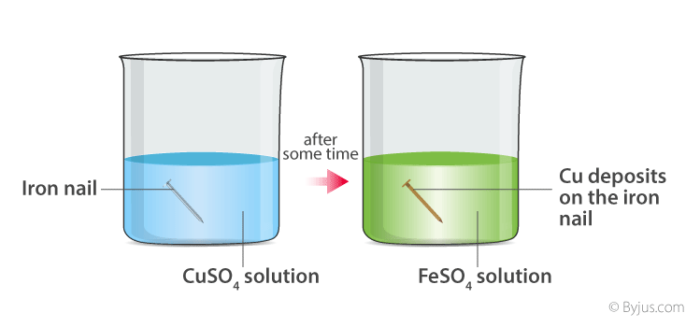
Physical change – change in colour or state occurs, but no new substance is formed.
Example: Water changes to steam on boiling, but no new substance is formed (Even though steam and water look different when they are made to react with a piece of Na, they react the same way and give the exact same products). This involves only a change in state (liquid to vapour).
To know more about Physical and Chemical Changes, visit here.
Students can refer to the short notes and MCQ questions along with separate solution pdf of this chapter for quick revision from the links below:
- Chemical Reactions and Equations Short Notes
- Chemical Reactions and Equations MCQ Practice Questions
- Chemical Reactions and Equations MCQ Practice Solutions
Observations that Help Determine a Chemical Reaction
A chemical reaction can be determined with the help of any of the following observations.
a) Evolution of a gas
b) Change in temperature
c) Formation of a precipitate
d) Change in colour
e) Change of state
Chemical Reaction
Chemical reactions are chemical changes in which reactants transform into products by making or breaking bonds (or both) between different atoms.
A chemical reaction is a process that causes one set of chemical components to change into another. Chemical reactions are defined as changes in the locations of electrons in the formation and breaking of chemical bonds between atoms, with no change in the nuclei, and are described using a chemical equation. At a given temperature and chemical concentration, chemical reactions occur at a predictable rate. Reaction speeds often increase as the temperature rises because more thermal energy is available to attain the activation energy required to break bonds between atoms.
Types of Chemical Reactions
Taking into consideration different factors, chemical reactions are grouped into multiple categories.
A few examples are:
● Combination
● Decomposition
● Single Displacement
● Double displacement
● Redox
● Endothermic
● Exothermic
● Precipitation
● Neutralisation
To know more about Chemical Reactions, visit here.
Chemical Reactions and Equations I
Word Equation
A word equation is a chemical reaction expressed in words rather than chemical formulas. It helps identify the reactants and products in a chemical reaction.
A chemical reaction is described using a word equation, which is a shorthand manner of expressing it. The names of the reactants are shown on the left side of a word equation. If there is more than one reactant, the names of the reactants are separated by a plus sign (+). Products are shown on the right side of a word equation. If there is more than one product, the names of the products are separated by a plus sign (+).
For example,
Sodium + Chlorine → Sodium chloride
The above equation means: “Sodium reacts with chlorine to form sodium chloride.”
Symbols of Elements and Their Valencies
A symbol is a chemical code for an element. Each element has a one or two-letter atomic symbol, which is, in most cases, the abbreviated form of its name.
Valency is the combining capacity of an element. It can be considered as the number of electrons lost, gained or shared by an atom when it combines with another atom to form a molecule.
Writing Chemical Equations
Representation of a chemical reaction in terms of symbols and chemical formulae of the reactants and products is known as a chemical equation.
![]()
• For solids, the symbol is “(s)”.
• For liquids, it is “(l)”.
• For gases, it is “(g)”.
• For aqueous solutions, it is “(aq)”.
• For gas produced in the reaction, it is represented by “(↑)”.
• For precipitate formed in the reaction, it is represented by “(↓)”.
To know more about Chemical Equations, visit here.
Balancing of a Chemical Reaction
Law of Conservation of Mass
According to the Law of Conservation of Mass, no atoms can be created or destroyed in a chemical reaction, so the number of atoms for each element on the reactants side has to balance the number of atoms that are present on the products side.
In other words, the total mass of the products formed in a chemical reaction is equal to the total mass of the reactants participating in a chemical reaction.
Balanced chemical equation
The chemical equation in which the number of atoms of each element on the reactants side is equal to that of the products side is called a balanced chemical equation.
To know more about Balanced Chemical Equations, visit here.
Steps for Balancing Chemical Equations
The changes that occur during a chemical reaction are represented by a chemical equation.
Reactants → Products
The equilibrium of all chemical equations must be maintained. This means that on both sides of the arrow, the number of each sort of atom must be the same.
Chemical equations are balanced using coefficients. A coefficient is a numerical value that is added to the front of a chemical symbol or formula. It indicates the number of atoms or molecules of the material involved in the process.
Place coefficients in front of symbols or formulas as needed to balance a chemical equation so that the same number of each type of atom appears in both reactants and products.
For example,
Zn + HCl → ZnCl2 + H2
The balanced equation is
Zn + 2HCl → ZnCl2 + H2
Hit and trial method: While balancing the equation, change the coefficients (the numbers in front of the compound or molecule) so that the number of atoms of each element is the same on each side of the chemical equation.
Comments
Post a Comment